Michael Rexach
| Professor of MCD Biology B.S., Syracuse University Ph.D., University of California, Berkeley Postdoctorate, Rockefeller University, 1993-1997 |
The Nuclear Pore Complex
Our work centers on elucidating the structure and function of the nuclear pore complex (NPC). This is the supramolecular porin structure in the nuclear envelope of all eukaryotes that creates the aqueous channel connecting the cytoplasm and nucleoplasm of the cell. Its main function is to serve as a size-selective gate in the vital flow of macromolecules between these compartments. It is composed of proteins termed nucleoporins, and approximately 450 of them are needed to form each NPC.
We have discovered that a defined subset of nucleoporins are natively unfolded (intrinsically disordered), yet can interact with each other to form a quaternary structure held together by weak hydrophobic attractions. Indeed, this network of FG domains functions in vivo as the physical element of the size-selective diffusion barrier in the NPC.
We are currently characterizing the dynamic behavior of these natively unfolded nucleoporins and their structural arrangement within the NPC transport conduit using combination of NMR spectroscopy and molecular modeling, guided by an extensive evolutionary analysis of the nucleoporin family in four Saccharomyces strains.
Please follow this link to find the lab's publications in the National Library of Medicine's PubMed database.
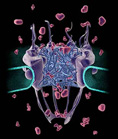
Model for the Nuclear Pore complex. Nuclear membrane is in cyan, the nuclear basket is at the bottom and the cytoplasmic fibrils are at the top. A meshwork of natively unfolded Nup proteins fills the center of the pore and creates a diffusion barrier for large proteins. For details, see Patel et al. (2007) Cell 129:83-96.
