Lindsay Hinck
 |
Distinguished Professor of MCD Biology B.S., Western Washington University M.S., UC Davis Ph.D., Stanford University Postdoctorate, UC San Francisco |
Cellular Interactions During Organogenesis and Tumorigenesis
My colleagues and I are interested in how epithelial cells assemble into organs during development, and how the reverse process occurs during cancer when cells disassemble and metastasize to inappropriate locations. We study two families of positional cues, called Slits and Netrins that were originally identified in the nervous system where they direct the construction of elaborate networks of neuronal connections. We focus on how these cues control the development of the mammary gland, and how loss of these cues during tumor progression contributes to breast cancer. Currently, the laboratory has projects in three areas.
Building an organ: The mammary gland is an elaborate tree-like epithelial structure, comprising an outer layer of myoepithelial cells and an inner layer of secretory luminal epithelial cells. We discovered new roles for Slit2 and Netrin1 in mediating the interactions between these two epithelial cell layers. Now we are investigating how these cues signal through their respective receptors to generate cell contact that is at once flexible and strong.
Stem cells and self renewal: The mammary gland is remarkable in its capacity to self renew since every pregnancy results in prodigious cell growth to prepare for milk production. Stem cells required to maintain this process reside in specialized environments (niches) squeezed between the luminal and myoepithelial cells layers. We are exploring how loss of Slits and Netrins disrupts these niches, and the biological consequences of this disruption.
Loss of growth control and cancer: One in eight women in the United States will develop breast cancer in her lifetime. Only about 15% of these cancers have been linked to specific gene mutations; therefore a major challenge in breast cancer research is to identify the causes of the disease. We have identified Slits as breast tumor suppressors that regulate several critical pathways controlling cell proliferation and migration. Current research focuses on developing therapeutic strategies to target these pathways.
Please follow this link to find the lab's publications in the National Library of Medicine's PubMed database.
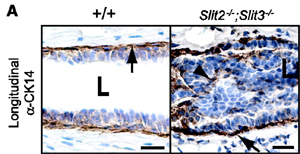
Loss of Slit2 and Slit3 expression in mammary epithelium leads to the formation of hyperplastic disorganized lesions. For details see Marlow et al. (2008) Cancer Research 68, 7819-7827.
