Bill Saxton
| Professor of MCD Biology B.S., Microbiology, University of Minnesota Ph.D., Molecular, Cell and Developmental Biology, University of Colorado, Boulder |
Organelle Transport and Neurodegeneration
The Saxton lab studies mechanisms that drive intracellular transport and cytoplasmic organization, using Drosophila as a model organism. To generate and maintain proper cytoplasmic order and thus their complex functions, cells use microtubules and force-generating motor proteins to transport RNAs, proteins, mitochondria and other organelles to appropriate locations. Neurons are especially dependent on such microtubule-based cytoplasmic transport, because their signaling functions rely on extraordinarily long cytoplasmic extensions (axons and dendrites) that require import of many components from their cell bodies. Defects in microtubule-based transport can cause or contribute to Alzheimer's, ALS, SBMA, spastic paraplegia and other neurodegenerative diseases.
We are currently focusing on how motor proteins accomplish their normal transport jobs in neurons and oocytes, and on identifying the cellular mechanisms that link defective transport to axon degeneration in our Drosophila spastic paraplegia model system. Our strategies usually begin with genetic screens, candidate gene perturbations, or chemical tests of microtubule-based transport processes, looking for specific motor-cargo interactions and ways to control those interactions. A central part of or analyses includes developing methods for high resolution tracking and quantification of single organelle movements in oocytes, neurons, and glial cells in live animals or tissues.
Please follow this link to find the lab's publications in the National Library of Medicine's PubMed database.
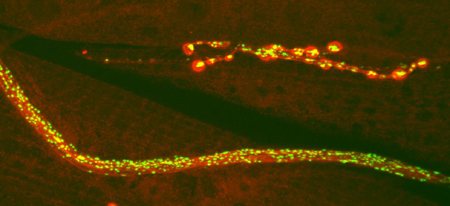
GFP-neuropeptide (green) and anti-syntaxin staining (red) reveal Drosophila nerves, muscles, and branched neuromuscular junctions.
